Biochemical constraints of salt stress
Physiological & Biochemical constraints of salt stress
4. Impact on plant growth
Salts in the soil water inhibit plant growth for two reasons.
- First, the presence of salt in the soil solution reduces the ability of the plant to take up water, and this leads to reductions in the growth rate. This is referred to as the osmotic or water-deficit effect of salinity.
- Second, if excessive amounts of salt enter the plant in the transpiration stream there will be injury to cells in the transpiring leaves and this may cause further reductions in growth. This is called the salt-specific or ion-excess effect of salinity.
- The salt in the soil solution (the ‘osmotic stress’) reduces leaf growth and to a lesser extent root growth, decreases stomatal conductance and thereby photosynthesis.
- The cellular and metabolic processes involved are in common with drought-affected plants.
5. Photosynthesis and Stomatal Conductance
- The most dramatic and readily measurable whole plant response to salinity is a decrease in stomatal aperture.
- Stomatal responses are undoubtedly induced by the osmotic effect of the salt outside the roots.
- Salinity affects stomatal conductance immediately, firstly and transiently owing to disturbed water relations and shortly afterward owing to the local synthesis of ABA.
- Factors decreasing photosynthetic rate due to salinity :
(1) Dehydration of cell membranes which reduce their permeability to CO2. High salt concentration in soil and water create high osmotic potential which reduces the availability of water to plants. Decrease in water potential causes osmotic stress, which reversibly inactivates photosynthetic electron transport via shrinkage of intercellular spaces.
(2) Salt toxicity caused particularly by Na+ and Cl– ions:
Cl- inhibits photosynthetic rate through its inhibition of NO3-/N uptake by the roots.
NO3-/N found to be significantly reduced in salt-stressed plants and this reduction was correlated with photosynthetic reduction.
The reduced NO3-N uptake combined with osmotic stress may explain the inhibitory effect of salinity on photosynthesis.
(3) Reduction of CO2 supply because of the closure of stomata:
The reduction in stomatal conductance results in restricting the availability of CO2 for carboxylation reactions.
At high salinity, salts may build up in the apoplast and dehydrate the cell, they may build up in the cytoplasm and inhibit enzymes involved in carbohydrate metabolism, or they may build up in the chloroplast and exert a direct toxic effect on photosynthetic processes.
Salinity induces K and Ca deficiency in the photosynthetic cells and decline in the level of pigmentation, results in inhibition of photosynthetic activity.
6. Disturbance to respiration
- A short-term decrease in transpiration rate is considered to be due to a transient increase in the membrane permeability for Na ions.
- An increase in the cellular demand for energy to expel Na ions may be the main cause of increase in the dark respiration.
- During the long-term exposure to hyper-salinity, an overall increase in the rate of respiration may be owing to the enhanced cytochrome oxidase and glucose 6-phosphate dehydrogenase activities.
Three Distinct Types of Plant Response or Tolerance
- The mechanisms of salinity tolerance fall into three categories
1.Tolerance to osmotic stress. The osmotic stress immediately reduces cell expansion in root tips and young leaves and causes stomatal closure.
A reduced response to the osmotic stress would result in greater leaf growth and stomatal conductance, but the resulting increased leaf area would benefit only plants that have sufficient soil water.
- Greater leaf area expansion would be productive when a supply of water is ensured such as in irrigated food production systems, but could be undesirable in water-limited systems, and cause the soil water to be used up before the grain is fully matured
- Na+ exclusion from leaf blades. Na+ exclusion by roots ensures that Na does not accumulate to toxic concentrations within leaves. A failure in Na+ exclusion manifests its toxic effect after days or weeks, depending on the species, and causes premature death of older leaves.
- Tissue tolerance, i.e., tolerance of tissue to accumulated Na+, or in some species, to Cl−.
Tolerance requires compartmentalization of Na+ and Cl− at the cellular and intracellular level to avoid toxic concentrations within the cytoplasm, especially in mesophyll cells in the leaf.
Toxicity occurs with time, after leaf Na+ increases to high concentrations in the older leaves.


1. Tolerance to osmotic stress
Osmotic adjustment
To maintain the normal growth, plants must readjust to increased external osmolality. This can be done by accumulating a variety of molecules in cytoplasm to counteract the external osmotic pressure.
Three major avenues are available for organisms
(i)Plants can accumulate a range of organic osmolytes (so-called compatible solutes) by
increasing their uptake from external media.
(ii) Osmotic adjustment can be achieved by de novo synthesis of compatible solutes.
(iii)Plants can rely on inorganic rather then organic osmolytes and increase accumulation
of Na+, Cl− and K+ for osmotic adjustment purposes.
Uptake of organic osmolytes is the most preferred option.
Four major classes of osmolytes are usually distinguished sugars, polyols, amino acids and quaternary ammonium compounds.
According to the classical view, accumulation of these non-toxic (thus compatible) osmotically active solutes will result in an increase in cellular osmolarity leading to the influx of water into, or at least reduced efflux from cells, thus providing the turgor necessary for cell expansion
Osmotic signal transduction pathway

Figure 2. Transduction of osmotic signal of salt stress through ABA-dependent and independent pathways (adapted from Taiz & Zeiger, 2011 and Shinozaki et al., 2015)
Osmotic signal transduction
- The large majority of osmotic component signal transduction-triggered genes are regulated by ABA, but some are not–suggesting that there are distinctive molecular regulatory mechanisms modulating genes responsive to salt stress in an ABA-dependent or independent regulatory pathway.
- Both pathways activate regulatory proteins (transcription factors), which interact with promoter region of specific genes up or down-regulating their expression (Fig. 2).
- ABRE is the main cis-acting regulatory element in ABA-responsive gene expression that requires for function another cis-acting element.
- Under salinity, the major ABA signaling cascades function upstream of basic leucine zipper (bZIP) transcription factors (for instance AREB and ABF), involving PYR1/PYLs/ RCARs receptors, PP2C phosphatases, and SnRK2 protein kinases (Shinozaki et al., 2015).
- Yet in the ABA-independent pathway, the promoters of genes contain a different cis-acting element, the DRE (dehydration-responsive element)/ CRT (C-Repeat). ABA-independent signaling cascades may include DREB2 transcription factors and/or a participation of MAPK signaling pathway (Taiz & Zeiger, 2011).
- Several genes are up-regulated by ABA-dependent and ABA-independent pathways in response to osmotic stress, including
- ion transporters
- regulatory proteins (transcription factors, protein kinases and phosphatases)
- stress-tolerance proteins, such as enzymes from antioxidative system and those involved in the synthesis of compatible solutes.
2. Regulation of Na+ homeostasis in roots and shoots
- Ions loaded into the root xylem are transported to the shoot largely by mass flow.
- Several processes are involved, including the regulation of Na+ transport into the shoot, and the reduction of Na+ content in the shoot by recirculation through the phloem back to the root, some halophytes use glands as salt sinks.
- These processes function to restrict cytotoxic ion accumulation into cells.
- A control response is to lower transpiration by a reduction in stomata aperture; however, this is only effective as a short-term response because plants need to maintain water status, carbon fixation and solute transport
Sodium accumulation & exclusion from roots
- Non-selective cation channels, NSCC
- NSCC are believed to be the major route for Na+ uptake into the root, and are gated by numerous factors such as cytosolic or external Ca2+ and pH levels, cyclic nucleotides, ATP, glutamate, G-proteins, ROS, and mechanical tension on membrane.
- The high affinity potassium transporter, HKT1
- HKT-type transporters, many of which seem to be sodium-specific, may be regulators of K+ homeostasis in the presence of Na+.
- The low affinity cation transporter, LCT1
- However, so far this transporter has been found exclusively in wheat species and, thus, is unlikely to represent a major pathway for Na+ entry into the root.
- A bypass flow, resulting from Na+ ‘leakage’ into the root via the apoplast.
- This uptake usually takes place in the mature root zone, where the integrity of the Casparian strip in the root endodermis is ruptured by protruding lateral roots. The extent of the contribution of this apoplastic flow to total Na+ influx into the plant varies dramatically between species.
- The main site of Na+ toxicity for most plants is the leaf blade, where Na+ accumulates after being deposited in the transpiration stream, rather than in the roots.
- A plant transpires 50 times more water than it retains in leaves, so excluding Na+ from the leaf blades is important.
- Most Na+ that is delivered to the shoot remains in the shoot, because for most plants, the movement of Na+ from the shoot to the roots in the phloem can likely recirculate only a small proportion of the Na+ that is delivered to the shoot.
- Controlling ion load into the root xylem restricts accumulation in the shoot to a level where cells in this organ can be effective ion repositories by vacuolar compartmentalization.
- Endodermal cells constitute a major control point in ion transport from the soil solution to the root xylem since the Casparian strip is an impermeable barrier to apoplastic solute movement.
- However, bypass systems that function through ‘leaks’ in the Casparian strip barrier or movement through areas of the root where the specialized endodermal cells are not fully developed may be additional major entry points.
- Regardless, vacuolar compartmentalization in cells that form the interconnected network between the soil solution and the root xylem progressively lowers the ions content that are entering the transpirational stream.
- The net delivery of Na+ to the xylem can be divided into four distinct components
- 1. Influx into cells in the outer half of the root;
- 2. Efflux back out from these cells to the soil solution;
- 3. Efflux from cells in the inner half of the root to the xylem; and
- 4. Influx back into these cells from the xylem before the transpiration stream delivers the Na+ to the leaf blade.



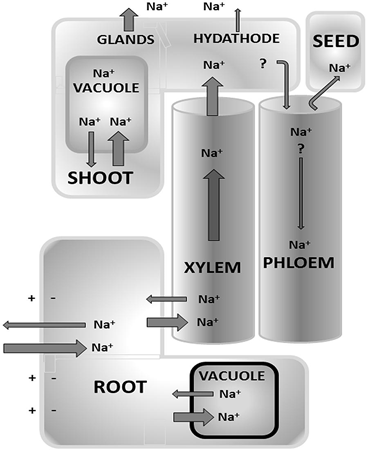
FIGURE . Overview of the main Na+ flux pathways that occur in terrestrial plants. Some mechanisms are still debated such as recycling of Na+ from shoot to root via the phloem.
Other mechanisms are anatomic adaptations found in a limited number of (halophytic) species only, such as extrusion via glands and hydathodes.
The size of the arrow only provides a relative measure of various fluxes but is not meant to be quantitative.

